Traditional Drug Delivery
Drug distributes via systemic
circulation with low/no specificity.
Target cells are often not reached
due to biological barriers.
NaDeNo’s Nanotechnology Platform
NaDeNo is re-defining drug delivery,
achieving high specificity in
hard-to-reach tissues/cells with:
- Local administration
- Tissue-adhesive nanoparticles carrying the drug
Engineered for Safety and Superior Drug Delivery
Differentiated for Impact
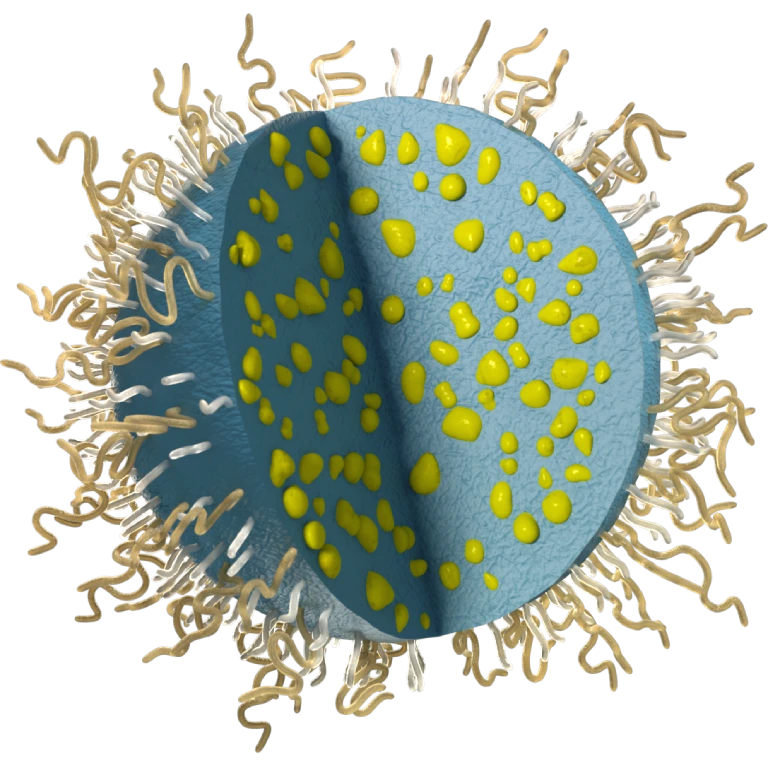

Drug is encapsulated and injected locally
Delivers drug safely to the target site, bypassing biological barriers.
Nanoparticles anchor directly to tissue
Local retention due to passive chemical mechanisms.
Drug is released precisely at the target site
Optimized drug release mechanism ensures sustained therapeutic levels locally while minimizing systemic toxicity.
Proprietary Materials. One-step Manufacturing Process.
Stable suspension,
long term stability
Sustainable & low cost
No downstream
processing needed
Our lead candidate, PACAB-002, will showcase our drug delivery platform
Lead Candidate
PACAB-002 (cabazitaxel nanoparticles)
Designed to target intraperitoneal micrometastases
Our innovative cabazitaxel nanoparticle formulation is engineered to reach, attach to, and destroy peritoneal micrometastases from ovarian cancer.
PACAB-002 polymer nanoparticle
– patented nanoformulation of cabazitaxel.
Cabazitaxel (encapsulated drug)
is FDA and EMA approved.
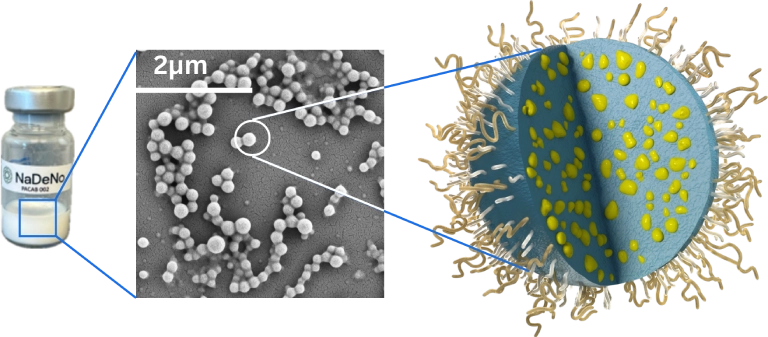
Ready-to-use Liquid Dispersion
Stable, homogenous formulation for precise and efficient dosing.

200 nm
The hidden reason why ovarian cancer
patients relapse
After initial cancer treatment, tiny tumor deposits (micrometastases) remain in the peritoneal cavity. Because they lack blood vessels, they were never reached by systemic treatment. This drives relapse and poor survival rates.
Why traditional therapy misses micrometastases
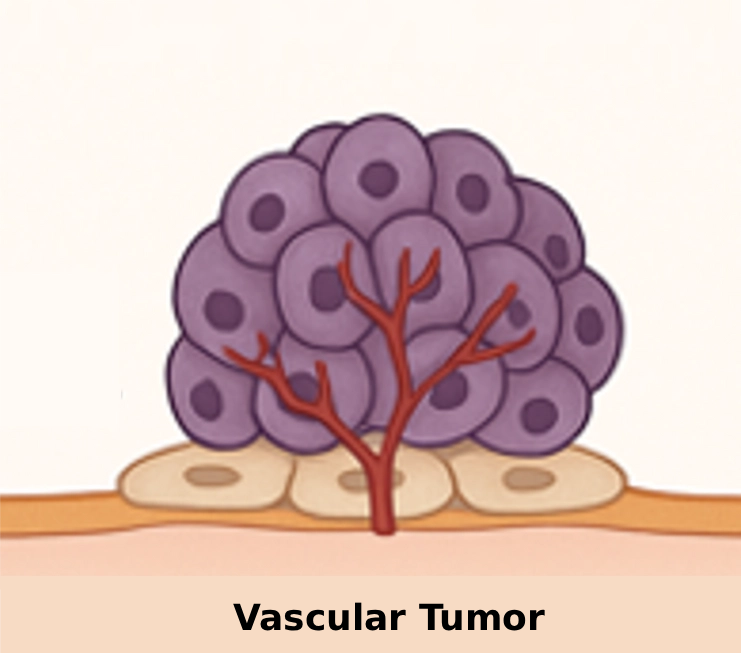
Tumors with blood vessels are reached through systemic drug administration.
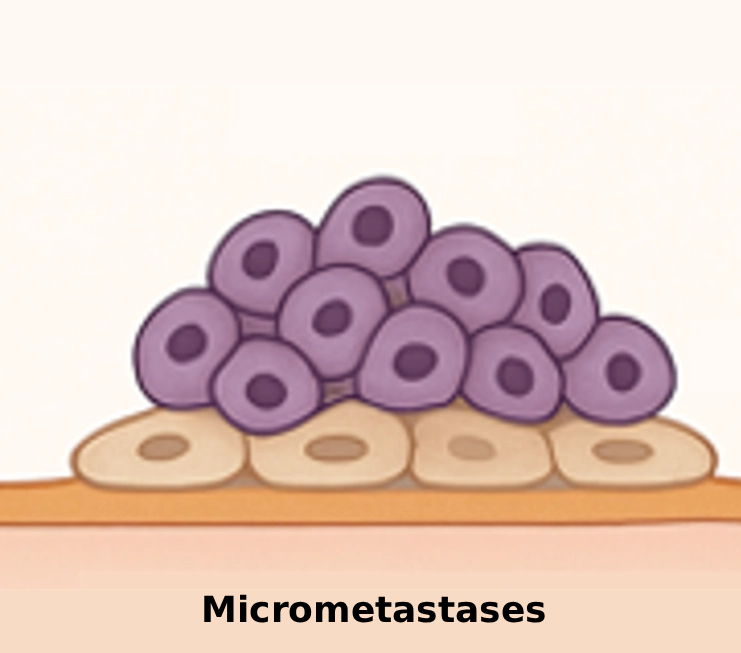
No blood vessels, so systemic drugs can’t reach them.
10x
Higher drug concentration in target cells
PACAB-002 achieves 10x higher drug concentration in tumors compared to free (non-encapsulated) drug.
97%
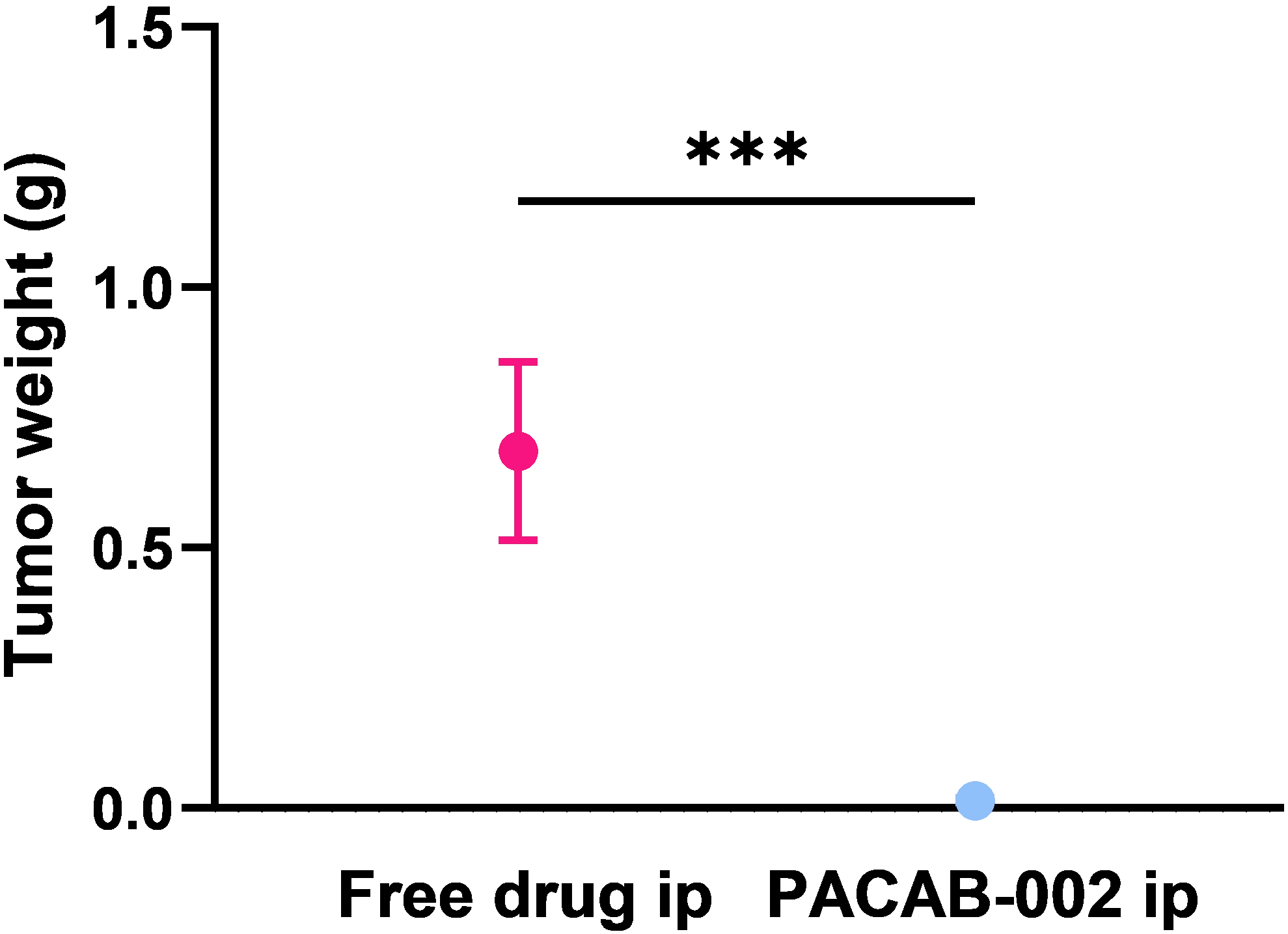
Tumor reduction with single dose
PACAB-002 eradicated tumors within 38 days with >97% tumor reduction compared to free drug.
100%
Survival after two cycles
100% survival after 2 treatment cycles. Median survival doubled with single dose vs. free drug.
Tumor-specific accumulation
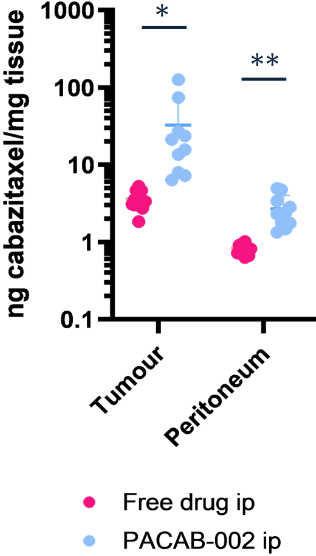
Tumor weight reduction
Survival
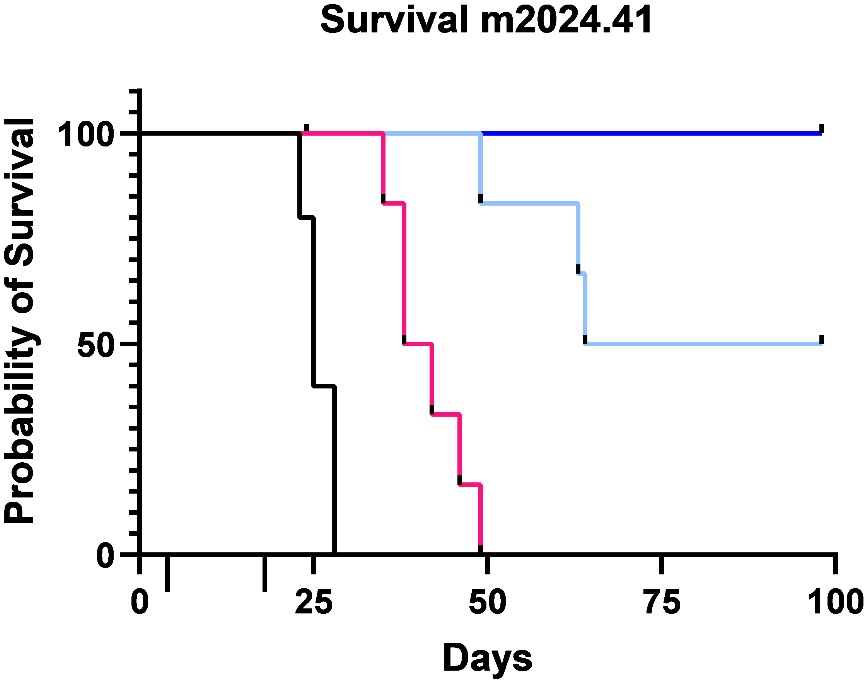
All data shown below are from the same ovarian cancer mouse model (IP administration, N=6).
Partnerships
A few of our many
partners across:
Innovation & Testing
Organizations that contribute to preclinical research, early testing, and technology development.
Clinical Development
Renowned clinical institutions driving patient-focused trials to validate efficacy and safety in real-world settings.
Manufacturing & Supply Chain
Partners enabling production, scalability, and raw material sourcing.
Funding & Ecosystems
Organizations providing financial support, innovation clusters, and strategic networks.
Regulatory & Intellectual Property
Experts in navigating regulatory approvals, patent protection, and compliance.














We’re excited to announce our newest partnership with AstraZeneca BioVentureHub
Insightful reads

What you’ll learn in this article:
- PACA drug encapsulation improves treatment response in peritoneal metastases models.
- Intraperitoneal injection of PACA nanoparticles provides a beneficial drug biodistribution.
- Drug encapsulation in PACA nanoparticles improves intraperitoneal drug retention.
- The taxane cabazitaxel is a promising chemotherapy option for peritoneal metastases.

What you’ll learn in this article:
This article from Norwegian SciTech News will provide deeper understanding of NaDeNo’s innovative nanoparticle-based drug delivery system.